A common question in materials science is whether Titanium electrodes can generate electricity on their own. The short answer is no—titanium itself is not a generator. It is a conductive material, not a fuel source.
However, the long answer is much more fascinating. Titanium electrodes are the critical “engine parts” within complex electrochemical systems. They do not create energy from nothing, but they are essential facilitators that allow us to convert chemical energy or light energy into usable electricity.
This article explores the mechanics of how titanium functions in energy systems, from flow batteries to advanced solar technology.

What Do Titanium Electrodes Actually Do?
In an electrochemical setup, titanium acts as the current collector or the reaction surface. It can function as either an Anode (positive) or a Cathode (negative) in various devices:
Electrolysis: Breaking down water or chemicals.
Electroplating: Deposting metals.
Energy Storage: Batteries and fuel cells.
Why Choose Titanium?
Raw titanium is chosen not for its ability to create power, but for its ability to survive the process of making it.
Corrosion Resistance: It withstands harsh acids and alkalis where other metals would dissolve.
Mechanical Strength: It maintains structural integrity under pressure.
Enhanced Performance: Titanium is rarely used “bare.” It is usually coated with Mixed Metal Oxides (MMO) or Platinum to improve conductivity and catalytic activity, ensuring the electrode remains stable over long operational periods.
The Science: How "Power Generation" Works with Titanium
For “electricity” to be produced using titanium, it must be part of a complete circuit. The basic principle relies on Electrochemistry:
The System: You need a pair of electrodes (Titanium +/-), an electrolyte (a conductive solution), and an external circuit.
The Driving Force: There must be a stable Redox (Reduction-Oxidation) Potential Difference between the two poles.
The Output: When chemical reactions occur at the electrode surface, electrons are released, creating a current flow.
Note: If the system requires external energy input (like a power supply or sunlight), the titanium acts as a “Reaction Electrode,” converting that external energy into chemical potential or electrical current.
3 Key Applications in Energy Systems
Titanium is a star player in three major energy generation and storage technologies:
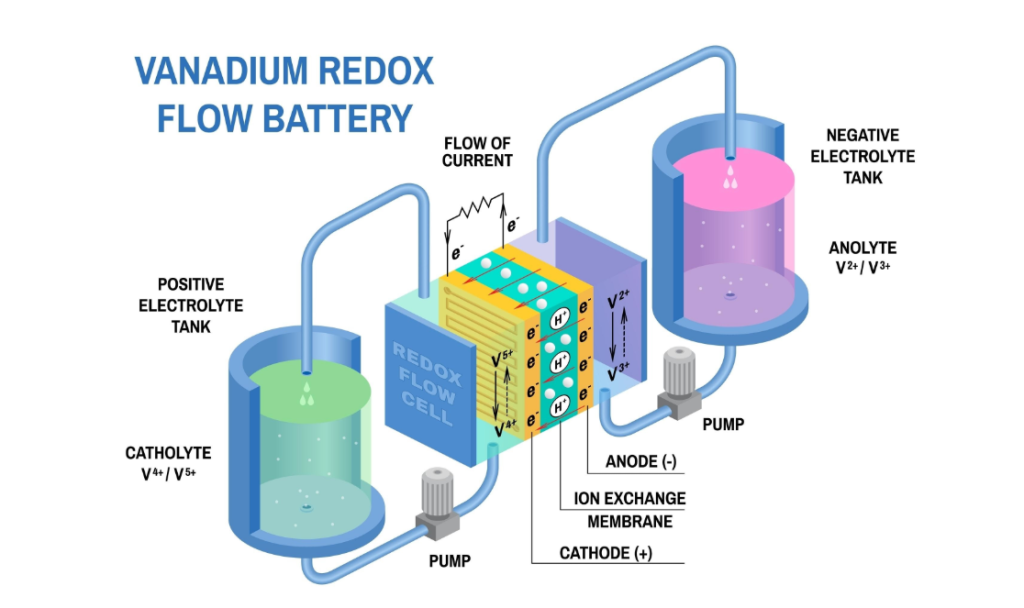
1. Redox Flow Batteries (Energy Storage)
In large-scale energy storage (like Vanadium Redox Flow Batteries), titanium is often used as the electrode material or the bipolar plate.
How it works: Titanium anodes and cathodes sit in electrolyte solutions containing metal ions of different valence states. As the ions oxidize and reduce, they release energy.
Titanium’s Role: It provides a stable surface for these reactions to occur without corroding in the acidic vanadium electrolyte.
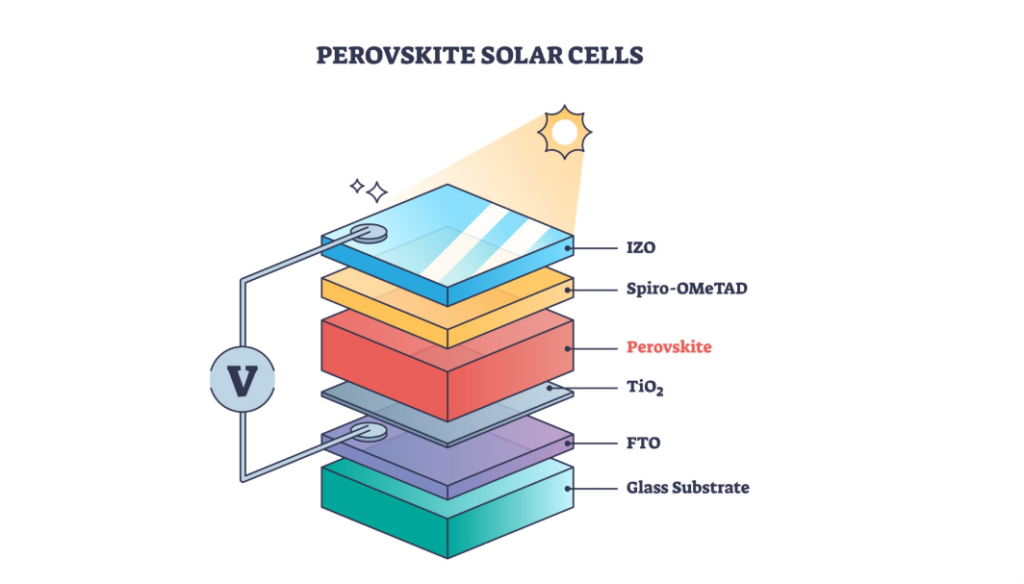
2. Photovoltaics (Solar Energy)
In next-generation solar technology, such as Perovskite Solar Cells or Dye-Sensitized Solar Cells (DSSC), titanium plays a microscopic but vital role.
How it works: A layer of Titanium Dioxide ($TiO_2$)—often a dense or mesoporous layer—acts as the Electron Transport Layer (ETL) or the electrode substrate.
Titanium’s Role: When sunlight hits the cell, it creates electron-hole pairs. The $TiO_2$ layer efficiently captures the electrons and directs them to the circuit to produce current.
3. Hydrogen Production (Indirect Generation)
While this is energy consumption initially, titanium electrodes are standard in water electrolysis to produce Hydrogen.
The Cycle: Titanium anodes split water into Hydrogen and Oxygen. This Hydrogen is then used in Fuel Cells to generate electricity later. Without the durability of titanium anodes, this green energy cycle would be inefficient and costly.
For the Hobbyist: Understanding the Experiment
If you are planning a DIY experiment to “generate electricity” with titanium, keep this in mind:
What WON’T work: Placing two identical titanium plates into a cup of tap water. Since the material and the environment are the same, the potential difference is near zero ($0V$). No current will flow.
What WILL work: You need a Chemical Potential Difference.
Use Titanium as one electrode and a different metal (like Copper or Zinc) as the other.
Use an electrolyte solution rich in ions (salt water or acidic solution).
Result: You will measure an Open Circuit Voltage (OCV) and can power a small load. However, the energy is coming from the chemical reaction of the system, not the titanium itself.
Titanium electrodes are not magical power sources, but they are the durable backbone of modern energy technology. Whether it is stabilizing a flow battery or transporting electrons in a solar cell, titanium enables the conversion of chemical and solar energy into the electricity that powers our world.
Next Step: Are you building a specific device, such as a Hydrogen Electrolyzer or a DIY Battery? Let me know your goal, and I can recommend the specific type of Titanium Electrode coating (e.g., Ruthenium-Iridium vs. Platinum) required for your project.



