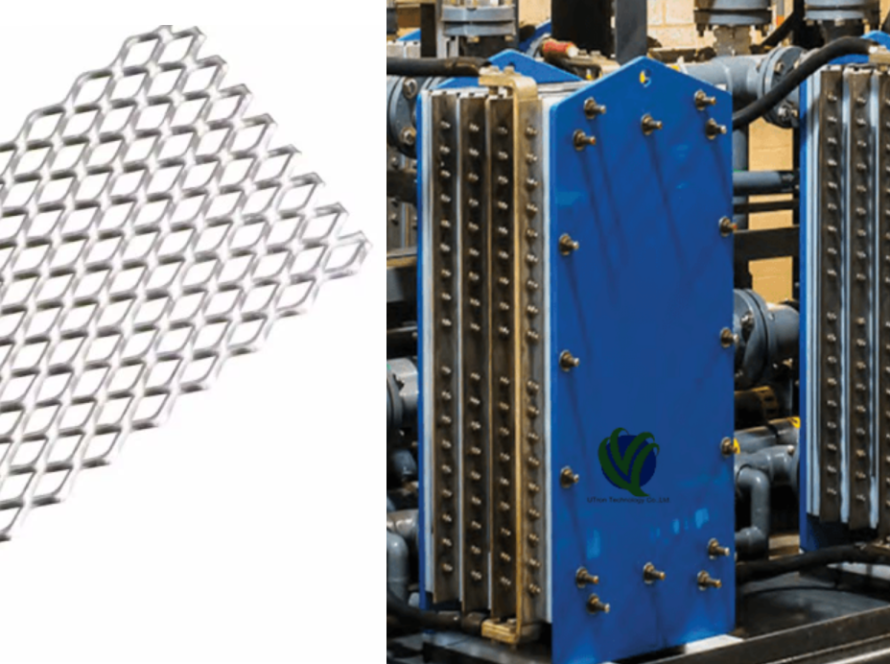
The Unique Advantages of the Titanium Anode: Why Choose a Titanium-Based Electrode?
The Titanium Anode is not an ordinary metal electrode; it usually refers specifically to an insoluble electrode with a titanium substrate coated with noble metals or metal oxides (such as DSA/MMO). The titanium substrate itself brings several superior characteristics unmatched by traditional materials:
Four Core Features
Exceptional Corrosion Resistance: Titanium forms a dense and stable oxide film in aggressive electrolytic environments (acid, alkali, salt). This allows the Titanium Anode to operate stably for extended periods in harsh processes like chlor-alkali and electroplating, far exceeding the lifespan of graphite or lead anodes.
Excellent Conductivity: Titanium is a good conductive material, and when paired with a high-performance noble metal coating (such as Platinum or $RuO_2$), it meets the required current density for various electrochemical reactions, ensuring efficient and uniform current distribution.
High-Temperature Stability: The inherent high melting point and superior mechanical properties of titanium allow the Titanium Anode to maintain structural integrity in high-temperature operating environments, preventing deformation or coating detachment.
Lightweight and High Strength: Titanium has low density but high strength. Using titanium electrodes not only reduces the overall weight of the equipment but also ensures the structural integrity and rigidity of the electrochemical device, making it particularly suitable for large industrial electrolytic cells.

The Precision Manufacturing Process of the Titanium Anode
The performance of the Titanium Anode is highly dependent on its manufacturing process—a precise procedure that transforms high-purity titanium raw material into a high-performance composite electrode.
Key Manufacturing Steps
Titanium Material Selection: High-purity, impurity-free industrial pure titanium or titanium alloy sheets are selected as the substrate, forming the foundation for superior final product quality.
Titanium Plate Processing and Shaping: The titanium plate is precisely cut, drilled, and mechanically processed according to the electrolytic cell design requirements (e.g., mesh, plate, or tubular shapes) to achieve the necessary geometry and dimensions.
Titanium Surface Pre-treatment: This is a crucial step, typically involving electrochemical or physicochemical methods like acid pickling (e.g., oxalic/hydrofluoric acid mixtures) to remove surface oxides and impurities. This process creates a rough, microporous structure to enhance the adhesion between the titanium substrate and the subsequent coating.
Noble Metal Coating (e.g., Platinizing): To impart electrocatalytic activity, one or more layers of noble metals or their oxides (e.g., $Pt$, $RuO_2$, $IrO_2$) are applied to the activated titanium substrate. This coating is the active part where the electrochemical reaction occurs, significantly reducing the reaction overpotential.
Assembly and Welding: Finally, the treated titanium plates are assembled into electrode packs according to design specifications, typically using precision welding methods like TIG welding, to ensure reliable conductive connections.
Widespread Applications: The Industrial Revolution Driven by the Titanium Anode
The high performance of the Titanium Anode has allowed it to replace traditional electrodes in several key sectors, becoming the core driver for improving efficiency and environmental standards.
Electrochemical Industry (High Energy Efficiency):
Chlor-Alkali Electrolysis: Manufacturing chlorine gas and caustic soda ($\text{NaOH}$), this is the earliest and largest application.
Water Electrolysis for Hydrogen Production: Efficiently electrolyzing water in cells to produce high-purity hydrogen and oxygen.
Metal Electrowinning/Electroplating: Providing a stable current environment in the extraction and plating of metals like copper, nickel, and zinc, minimizing soluble impurities from the anode, and improving product purity.
Industrial & Environmental Applications (Zero Contamination):
Wastewater Treatment and Desalination: Utilizing electrochemical oxidation to treat persistent industrial wastewater, degrading organic pollutants (COD); also used in electrodialysis for seawater desalination.
Water Disinfection: In hospitals, swimming pools, or cooling water systems, salt water is electrolyzed to produce sodium hypochlorite for disinfection, reducing the need for bulk chemical agents.
Cathodic Protection: Used as sacrificial or auxiliary anodes to protect underground pipelines, reinforced concrete structures, and marine facilities from corrosion.
High-Tech and Medical Fields (Lightweight, High Strength):
Aerospace: Due to its lightweight and high-strength properties, it is widely used in manufacturing high-tech equipment components for satellites and aircraft.
Medical Devices: The biocompatibility of titanium makes it suitable for manufacturing medical devices, including pacemaker casings, artificial joints, and bone plates.