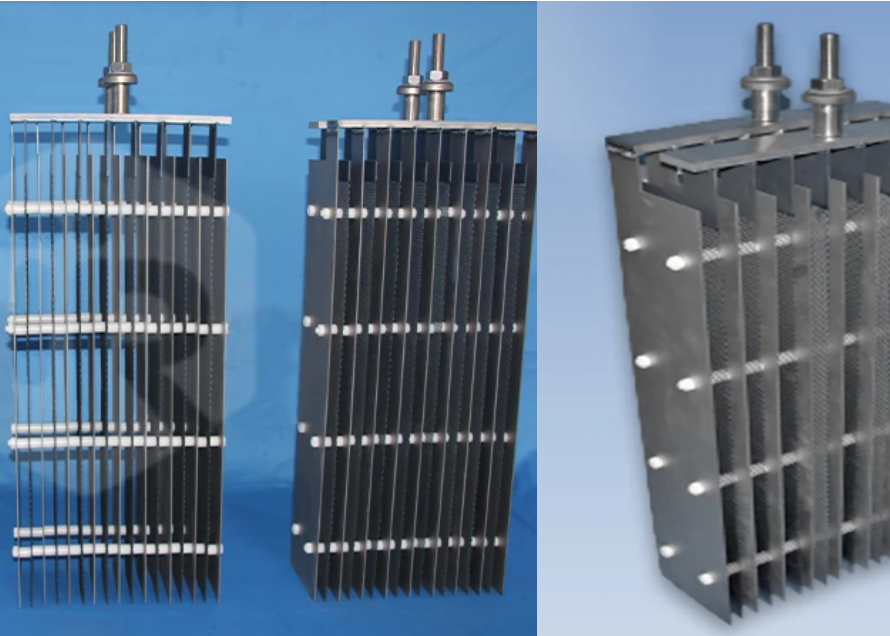
In modern electrochemical industries, if the electrolytic cell is the heart, the electrode is the pacemaker driving its beat. Among various electrode materials, the Titanium Anode ($\text{Ti Anode}$) has become an indispensable key component due to its outstanding performance.
Often referred to as “Dimensionally Stable Anodes” (DSA) or MMO anodes (Mixed Metal Oxide), the $\text{Ti Anode}$ has completely revolutionized sectors ranging from water treatment to metal extraction. It is not just a metal rod but a high-tech composite material that precisely combines a titanium substrate with a noble metal coating.
Next, we will deeply analyze its structure, working principles, and why it has replaced traditional materials to become the industry’s choice.
What is a Titanium Anode? The Dual Structure from Substrate to Coating
To understand the power of the titanium anode, we must first break down its “skeleton” and “soul.” It is not a single metal but a composite electrode material, mainly consisting of two parts:
The Substrate (The Skeleton): Industrial Pure Titanium
Titanium is chosen as the substrate for its extreme corrosion resistance. Its primary role is to provide mechanical support and a conductive path. In harsh, aggressive environments containing strong acids, bases, or chlorides, the titanium substrate remains structurally stable, unlike graphite or lead anodes, which are prone to corrosion or deformation.
\
The Coating (The Soul): Noble Metal Oxides
This is the core technology of the titanium anode. A very thin, yet highly active, layer of noble metal oxides (such as Ruthenium Oxide $RuO_2$, Iridium Oxide $IrO_2$, or Platinum $Pt$) is applied to the titanium surface via brushing or sintering processes. It is this coating, only a few micrometers thick, that undertakes the actual electrocatalytic reaction.
Expert Insight: You can imagine the titanium anode as a highway—the titanium substrate is the sturdy roadbed ensuring the road doesn’t collapse, while the noble metal coating is the high-performance asphalt surface, ensuring the current (traffic flow) passes quickly with minimal resistance.
Working Principles of the Titanium Anode: More Than Just Conduction
Many newcomers to the industry believe the anode is simply for conducting electricity, but at a microscopic level, the Titanium Anode is performing a complex electrochemical “operation.” Its working principle can be summarized on three levels:
1. Surface Electrocatalysis (The Key to Energy Saving)
The electrolysis process is essentially the transfer of electrons. On the anode surface, the MMO (Mixed Metal Oxide) coating acts as a catalyst.
It significantly lowers the reaction’s “overpotential” (the extra voltage required for the reaction).
This means that under the same current density, the cell voltage is lower when using a titanium anode, directly resulting in reduced electricity consumption.
2. Targeted Reaction Selectivity
Different coating formulations determine the anode’s “personality”:
Chlorine Evolution Type (Ruthenium-based): Primarily catalyzes the oxidation of chloride ions to generate chlorine gas ($2Cl^- \rightarrow Cl_2 \uparrow + 2e^-$), commonly used in brine electrolysis.
Oxygen Evolution Type (Iridium-based/Tantalum-based): Primarily catalyzes the electrolysis of water molecules to generate oxygen gas ($2H_2O \rightarrow O_2 \uparrow + 4H^+ + 4e^-$), commonly used in copper foil production or wastewater treatment.
3. Dimensional Stability Mechanism (The Origin of DSA)
This is the most famous characteristic of the titanium anode.
Traditional Anode Pain Points: Graphite anodes gradually oxidize and flake off during operation, leading to increased inter-electrode gap and unstable voltage.
Titanium Anode Advantage: Regardless of the operating duration, the titanium substrate is virtually insoluble, and the anode’s geometric size and shape remain unchanged. This ensures a constant inter-electrode gap, thereby maintaining a stable electric field distribution and cell voltage.
Why Does Industry Favor the Titanium Anode? Three Core Advantages
Compared to traditional lead or graphite electrodes, the initial investment for a Titanium Anode may be higher, but its comprehensive life-cycle benefit is extremely attractive.
1. Significant Energy Savings
Data show that in the chlor-alkali industry, the use of titanium anodes typically reduces DC power consumption by 10% – 20%.
Reason: The high catalytic activity of the coating reduces the gas evolution overpotential. For high-energy consumption electrolytic industries, the electricity savings often cover the anode cost in a short time.
2. Extended Service Life and “Zero Contamination”
Corrosion Resistance: With appropriate coating protection, titanium anodes can operate continuously for thousands to tens of thousands of hours.
Product Purity: Historically, when lead anodes were used, dissolving lead would contaminate the electrolyte and the cathodic product with heavy metals. The “insolubility” of the titanium anode completely solves this issue, which is crucial for producing high-purity metals (such as in electrowinning of copper and gold).
3. Supports High Current Density Operation
Traditional anodes are prone to overheating or disintegration at high currents, limiting production efficiency. The Titanium Anode, combined with an optimized coating, can withstand significantly higher current densities (e.g., boosting production from $2000 A/m^2$ to much higher levels). This means that within the same facility footprint, your output can be multiplied.
Typical Applications: From Chemical Giants to Home Pools
Thanks to its flexible coating adjustments, the Titanium Anode is active in a wide range of scenarios:
Chlor-Alkali Industry: Production of caustic soda and chlorine gas; this is the earliest and most mature application for titanium anodes.
Water Electrolysis Disinfection: Whether in water treatment plants or home swimming pool salt chlorinators, titanium anodes silently electrolyze salt to produce sodium hypochlorite for safe disinfection.
Environmental Wastewater Treatment: Utilizing high potential oxidation to degrade organic pollutants (COD) in wastewater, achieving harmless discharge.
Cathodic Protection: Used as an auxiliary anode in marine engineering or underground pipelines to prevent steel structure corrosion.

Are you looking for the right anode solution for your specific electrolysis project?
Are you looking for the right anode solution for your specific electrolysis project?
Titanium anodes are not a “one-size-fits-all” product. Different electrolyte media (sulfuric acid, chloride, or mixed systems) require matching noble metal coating formulations to achieve optimal results.
If you would like to know which coating system (e.g., Ruthenium-Iridium vs. Iridium-Tantalum) you should choose for your specific process (such as electroplating, wastewater treatment, or electrowinning), I can provide a detailed comparison of the technical parameters and applicable scenarios for different coatings. Would you like me to do that comparison for you?